View Project
Role of neoadjuvant high-dose proton pump inhibitors (PPI) and low-dose nivolumab with chemotherapy in breast cancer patients (PUNCH_BC): a phase II study.
Status: New
Lab/Organization
| Name & address of the Laboratory/Organization | Tata Memorial Centre | |
| Website address | https://tmc.gov.in | |
| Affiliated to which Department/Ministry | Department Of Atomic Energy (DAE) | |
| CSR Registration Number | CSR00001287 | |
| Registration under 12A | INS/12128 Provisional approval no.- AAATT3620RE20214 | |
| Registration under 80G | DIT(E)/ITO(Tech)/80G/2010-11 Provisional approval no.- AAATT3620RF20241 | |
| Name of the CSR Nodal | Dr Heena Shaikh | |
| Contact information of CSR Nodal | +91-22 -68735000 Extn: 5483 , hshaikh@actrec.gov.in | |
| Principal Investigator | Dr Prabhat Bhargava, bhargava611@gmail.com | |
| Co- Principal Investigator (Co-PI) | ||
Project Detail
| Objective on the basis of need | The trial aims to address the critical need for more effective and cost-effective treatment options for patients with HER2-positive and triple-negative breast cancer (TNBC), both of which are associated with limited cost-effective therapies or poor prognosis. By exploring the synergistic effects of high-dose PPIs and low-dose nivolumab in combination with standard chemotherapy, the project seeks to enhance treatment efficacy and improve patient outcomes. |
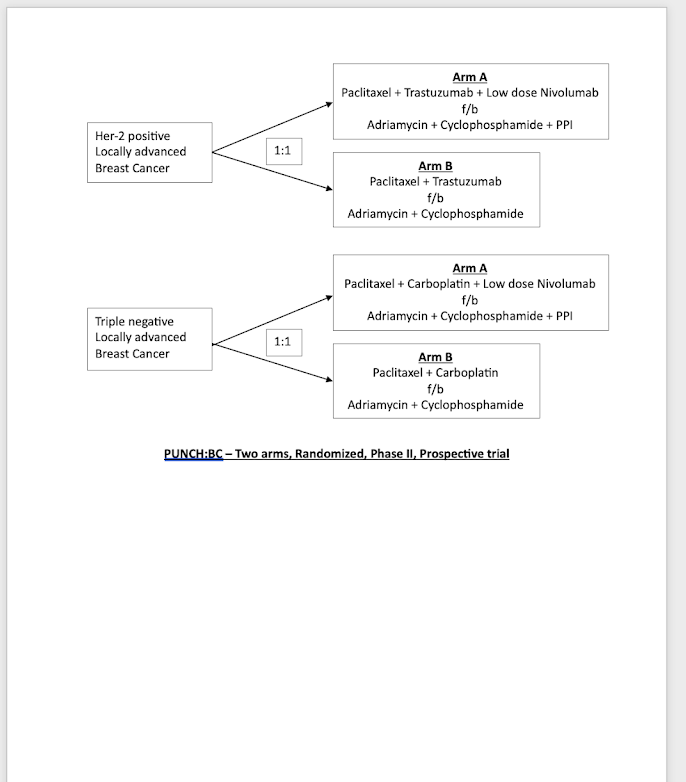
| Executive summary of the proposed project (In 250 words) | This is a phase II randomised study testing a new treatment strategy for breast cancer by combining chemotherapy, an acid-reducing medication called a proton pump inhibitor (PPI), and a low-dose of immunotherapy drug (nivolumab). The goal is to enhance the immune system’s ability to fight cancer while providing a more cost-effective and accessible treatment, especially for patients in India where full-dose nivolumab is often unaffordable. The trial targets two breast cancer types: 286 patients with triple-negative breast cancer (TNBC) and 280 patients with HER2-positive breast cancer. 1. Triple-Negative Breast Cancer (TNBC) cohort ● Arm A will receive weekly Paclitaxel and Carboplatin, with nivolumab (in alternate cycle). followed by dose-dense Adriamycin/Epirubicin, Cyclophosphamide, and PPI. ● Arm B: follows the same regimen but excludes nivolumab and Omeprazole. 2. HER2-positive cohort ● Arm A includes weekly Paclitaxel, Trastuzumab, and nivolumab (with Pertuzumab if feasible) followed by dose-dense Adriamycin/Epirubicin, Cyclophosphamide, and Omeprazole. ● Arm B: follows the same regimen but excludes nivolumab and Omeprazole. This strategy uses PPIs to enhance the efficacy of chemotherapy and to reduce the acidity in tumours, which helps stop cancer from growing and becoming resistant to treatment. At the same time, nivolumab strengthens the immune system so it can better attack cancer cells. Combining these treatments with chemotherapy aims to improve results. Currently, standard treatments like chemotherapy, surgery, and full-dose immunotherapy/targeted therapies are not feasible for many Indian patients. If successful, this trial could establish a cost-effective standard of care for HER2-positive and TNBC, enhancing survival rates, quality of life, and treatment options for patients with aggressive breast cancers. |
| Technology Readiness Level (If not a new project but an advancement of existing know how) | The trial represents an advancement of existing knowledge, combining known treatments (PPIs and nivolumab) with chemotherapy in a novel way for breast cancer therapy. |
| Outomes or Deliverables | The expected outcomes include improved disease-free survival rates, enhanced overall survival, a better understanding of the adverse event profile, and insights into quality of life impacts for patients undergoing this treatment regimen. |
| Project aligned with which most relevant UN SDGs | Goal 3 - Good Health & Well-Being Goal 9 - Industry, Innovation & Infrastructure |
| Duration (In years) | 6 years (3 years for patient recruitment and 3 years for follow-up). |
| Expected Impact | The anticipated impact includes ● Improved survival rates and quality of life for breast cancer patients. ● Reduction in treatment disparities, especially in resource-limited settings, due to cost-effective lowdose nivolumab. ● Enhanced understanding of combination therapy in cancer treatment. |
| Implementation model (self- implemented/ outsourced partnership) | The project will be self-implemented (investigator-initiated), with collaboration from clinical research teams and institutions involved in the study. |
| Total Budget (Recurring +Non-Recurring Expenses) | Rs 7,39,40,800/ |