View Project
A multicentre randomized study of neoadjuvant systemic therapy in oral cavity cancer
Status: New
Lab/Organization
| Name & address of the Laboratory/Organization | Tata Memorial Centre | |
| Website address | https://tmc.gov.in | |
| Affiliated to which Department/Ministry | Department Of Atomic Energy (DAE) | |
| CSR Registration Number | CSR00001287 | |
| Registration under 12A | INS/12128 Provisional approval no.- AAATT3620RE20214 | |
| Registration under 80G | DIT(E)/ITO(Tech)/80G/2010-11 Provisional approval no.- AAATT3620RF20241 | |
| Name of the CSR Nodal | Dr Heena Shaikh | |
| Contact information of CSR Nodal | +91-22 -68735000 Extn: 5483, hshaikh@actrec.gov.in | |
| Principal Investigator | Dr. Arjun Singh Assistant Professor and Surgeon, arjun193@gmail.com | |
| Co- Principal Investigator (Co-PI) | ||
Project Detail
| Objective on the basis of need | To compare the survival time without disease between the current standard treatment (surgery followed by radiotherapy +/- chemotherapy) and the experimental arm (adding low dose immunotherapy and chemotherapy before standard therapy of surgery) |
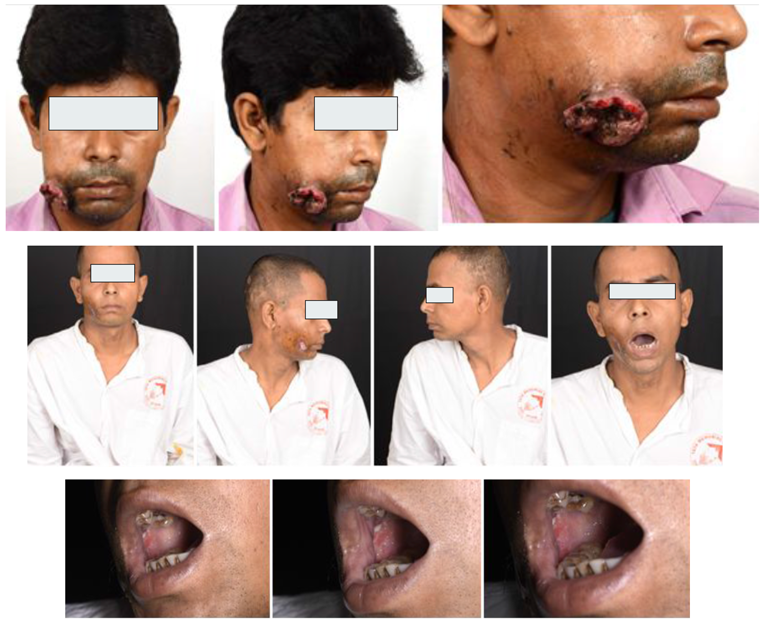
| Executive summary of the proposed project (In 250 words) | In India, oral cavity cancer is the most common cancer. Oral cancers cause huge burdens on health care systems in India, which are already under tremendous strain. Of the 1.3 lakh oral cancer patients diagnosed in India every year, majority present in locally advanced stage. There has been no new development in improving outcome of these patients in last three decades. In recent times, Immunotherapy has shown to improve outcome of these patients. Considering the cost limitation only, a small fraction of patients is able to take this treatment. There is urgent need to develop treatment, which is effective, accessible and easy to implement to majority of patients in our country. As the oral cavity cancer is the disease of developing country, there is limited research work in this area in western world. We have developed a cost-effective treatment (adding immunotherapy with traditional triple metronomic chemotherapy) in this setting while integrating Immunotherapy for these patients. This is a Phase 3 randomized trial. This will potentially improve survival for these patients. The sample size is 382 comparing Arm A – Surgery followed by RT or CTRT, and Arm B – Neoadjuvant chemotherapy with immunotherapy followed by Surgery with RT or CTRT. Almost around 1lakh, oral cavity cancer will benefit in India every year. This will also benefit oral cavity cancer patients in developing countries where immunotherapy is not affordable to most of the patients today. This includes SAARC region countries, Majority of Asia, Africa, Part of middle east, South American countries |
| Technology Readiness Level (If not a new project but an advancement of existing know how) | The combination and dosage have been well established in the literature. We have published the work of this novel commination in major journals and are now testing it in a randomized clinical trial. |
| Outomes or Deliverables | Arm A – Surgery followed by RT or CTRT Arm B – Neoadjuvant chemotherapy with immunotherapy followed by Surgery with RT or CTRT Primary objective: To compare the disease-free survival Secondary objectives: a. To compare the overall survival b. To compare the adverse event rates c. To compare the cost utility and economic evaluation We will also be evaluating the bio samples for extensive testing to determine which tumors are responders or not. |
| Project aligned with which most relevant UN SDGs | Goal 3 - Good Health & Well-Being Goal 9 - Industry, Innovation & Infrastructure Goal 10 - Reduced Inequalities |
| Duration (In years) | 5 years approximately |
| Expected Impact | We expect to see an increased survival rate in the group that receives this novel combination of therapy. Almost around 1 lakh oral cavity cancer will benefit in India every year. This will also benefit oral cavity cancer patients in developing countries where immunotherapy is not affordable to most of the patients today. This includes SAARC region countries, Majority of Asia, Africa, Part of middle east, South American countries |
| Implementation model (self- implemented/ outsourced partnership) | Self-implemented with additional staff hired |
| Total Budget (Recurring +Non-Recurring Expenses) | Total Budget – 13.96 Crore (Budget for clinical work – 4.34 Crore, Budget for translational work – 9.62 Crore). Till now we have received fund of 59 lakh for the project (clinical part of the project) |